© 2003 American Heart
Association, Inc.
Clinician Update |
Contemporary Management of Patent Foramen Ovale
From the Swiss Cardiovascular Center Bern, University of Bern, University Hospital, Berrn, Switzerland (B.M.); and the Cardiology Department, Childrens Hospital, Boston, Harvard Medical School, Boston, Mass.
Correspondence to Bernhard Meier, MD, FACC, FESC, Professor of Cardiology, Chairman, Swiss Cardiovascular Center Bern, University Hospital, CH-3010 Bern, Switzerland. E-mail bernhard.meier@insel.ch
In 1877, Cohnheim performed a necropsy on a young woman who had died from a stroke. He hypothesized that a clot passing through the patent foramen ovale must have caused her demise.1 Thus, the first description in medical literature on paradoxical embolism appeared.
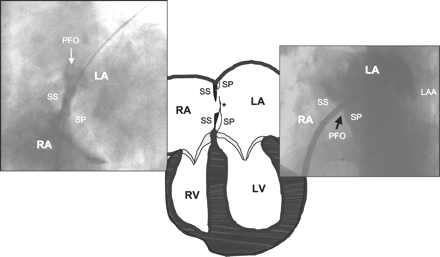
The foramen ovale is a pivotal feature during intrauterine life. As depicted in Figure 1, the interatrial septum primum on the left side and the interatrial septum secundum on the right side maintain a central hole after having grown from the periphery to the center. This hole is positioned caudally in the septum secundum and cranially in the septum primum, forming a slit valve that opens with pressure from the right. The blood from the umbilical vein entering through the inferior vena cava from the bottom of the right atrium keeps this door open until after birth. From then on, the left atrial pressure, slightly higher than the right atrial pressure, keeps the valve shut. In most individuals, the caudal portion of the septum primum on the left side and the cranial portion of the septum secundum on the right side fuse permanently, closing the foramen. In a minority of the population, however, the fusion does not take place and the foramen remains able to be opened (patent).
Prevalence of Patent Foramen Ovale
A pooled analysis of autopsy studies yielded an average prevalence of patent foramen ovale (PFO) of 26% (range 17% to 35%) (Table).2
Risultati degli studi clinici sul PFO
|
||||||||||||||||||||
In most echocardiographic studies on ischemic stroke patients, the prevalence of a PFO is higher in patients with a cryptogenic stroke. In a recent study of 61 patients, a PFO was found in 45% of those with cryptogenic stroke and in 23% of those with a stroke associated with large vessel atherosclerosis, lacunar ischemia, or cardiogenic embolism.3 This discrepancy is larger in young patients than in the elderly. Whereas the absolute risk of cryptogenic (including paradoxical) strokes increases with age, the relative stroke risk of a PFO is reduced as other etiologies become more dominant (Table).2 Some studies focusing on elderly populations have thus failed to reveal a relationship between PFO and stroke.
Clinical Problems Attributed to PFO
Paradoxical Embolism
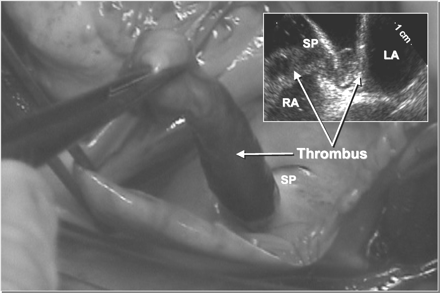
Although large thrombi may occasionally pass through the foramen ovale
(Figure 2),
this is more common for small clots of a few millimeters that
would normally embolize to the lungs and spontaneously lyze in
the lung filter without clinical sequelae. These clots will be
clinically recognized only if they paradoxically embolize to a
sensitive organ such as the brain, the eye, or the heart muscle
via the coronary arteries. The source of the clots cannot be established
in most patients; fewer than 10% will have deep-vein thrombosis
apparent on phlebography.4 Although
most emboli presumably arise from systemic veins, the PFO itself
has been suspected to be a source of thrombus because of stagnated
blood in the tunnel. However, the fact that, to our knowledge,
dislodging of such thrombi has not been reported during transcatheter
PFO closures argues against this hypothesis.
|
The percentage of cryptogenic strokes among ischemic strokes (about 75% of all strokes) varies from 8% to 44%, with a mean of 31% (Table).2 Assuming an annual incidence of 750 000 strokes in the United States,5 about 600 000 will be ischemic. Of these, about 200 000 will be cryptogenic, and of these roughly 70 000 will be associated with a PFO. By adding patients with transient ischemic attacks (TIA) or peripheral embolism, paradoxical embolism associated with a PFO identifies about 100 000 patients per year in the United States for whom closure of a PFO becomes an option. That amounts to roughly 10% of the yearly number of patients undergoing coronary angioplasty in the United States. Add to these the clinical syndromes discussed below, and the scope of PFO as a clinical problem can be grasped.
Decompression Illness in Divers
An increased prevalence of brain lesions has been found in divers even
in the absence of recognized decompression illness.6 In a
seminal study, transcranial Doppler ultrasonography detected a right-to-left
shunt in all divers with multiple brain lesions.7 A
foramen ovale no doubt accounted for most of these cases. A comparative
investigation regarding brain lesions and the presence of a foramen
ovale in sport divers and non-diving controls showed that brain
lesions were more common in individuals with a foramen ovale, although
divers had more brain lesions than non-divers, irrespective of the
presence of a PFO.8 This
has led some diving schools to recommend screening for the presence of
a PFO for professional divers or avid amateurs. In such divers, PFO
closure would make sense.
Migraine
The surprising results of a retrospective study in 37 patients with
percutaneous PFO closure for diving accidents or paradoxical embolism
revived interest about the association between migraine and PFO.9 Subsequently,
2 recent studies reported a 2- to 5-fold increased prevalence for
migraine in PFO carriers.10,11 The reason
for this apparent association between PFO and migraine remains undefined.
Small emboli or serotonin not metabolized in the lung were considered
as possible causes.
Miscellaneous
The risk of a PFO in the perioperative period has not been investigated systematically.
However, the increased presence of potential paradoxical emboli
(air, venous clots, or fat), in association with unphysiological
intrathoracic pressures (ventilation, open chest, straining, etc),
is of concern. It has been suggested that high-risk patients be
screened for PFO before susceptible surgery.12
In the context of pulmonary embolism, a 5-fold increased risk for mortality or systemic emboli was found in patients with a PFO.13
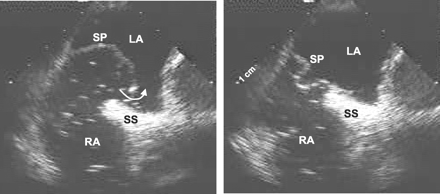
A rare and peculiar syndrome is platypnea orthodeoxia.14 It can be seen in elderly patients who become cyanotic and dyspneic while sitting up; these problems disappear when the patients are lying down. A right-to-left atrial shunt can be documented even in the absence of an elevated pressure in the right atrium. It is assumed that with aging a prominent Eustachian valve becomes redirected to the foramen ovale. Figure 3 explains this mechanism. This may be caused by general enlargement of the heart chambers and the aortic root or by a positional change of the entire heart due to obesity or spinal shortening. Associated pulmonary hypertension may lead to continuous arterial desaturation and cyanosis, irrespective of the patients upright or supine position.
|
Diagnosis of PFO
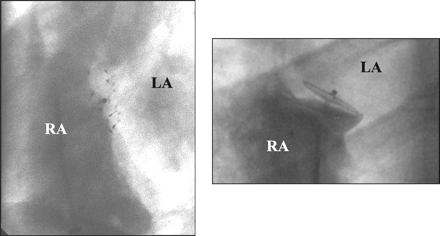
Although the PFO can occasionally be convincingly documented even
in adults with a transthoracic echocardiogram (TTE) (Figure 4), such
a diagnosis is rarely unequivocal. Transesophageal echocardiography
(TEE), rather than TTE, is the method of choice.15,16 A
bubble test with an aerated colloid solution at the end of a sustained
Valsalva maneuver (gush of blood filling the right atrium of the
empty heart several beats before the left atrium gets filled, thereby
opening the foramen) results in an excellent sensitivity provided
that the correct plane is visualized. Transcranial Doppler examination
is also very sensitive and specific.17 Likewise, indicator
dilution and pulse oximetry techniques have been validated and found
to have a sensitivity of 85% and 76%, respectively, whereas both
have a specificity of 100%.18 None
of these latter examinations can distinguish between a shunt at
the level of the PFO or elsewhere, however, nor do they give information on
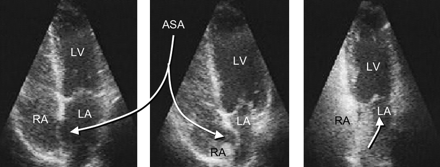
the presence or absence of an atrial septal aneurysm (Figure
5).
|
|
An atrial septal aneurysm (more appropriately called hypermobile or floppy septum primum) (Figure 5) was initially considered to be an independent risk factor for systemic embolism. Gradually it has been recognized as an accomplice of the PFO rather than a lone culprit. An atrial septal aneurysm without a PFO showed no risk for cryptogenic stroke in a TEE study of about 600 patients with cryptogenic stroke followed-up for 4 years.15
Rationale for PFO Closure in Stroke Patients
Studies on annual recurrences after a cerebral vascular accident (CVA)
or a TIA1924 reported
an incidence ranging from 3%23 to
16%.24 In
a large study, the recurrent stroke rate or mortality from an embolic
event was 6% to 8% per year.22 A
pooled analysis suggests that the presence of a PFO alone increased the
risk for recurrent events 5-fold, with an even higher risk in the
presence of an atrial septal aneurysm.16 Other
studies have found no significant influence of an isolated PFO but
show a strong influence by a PFO associated with an atrial septal aneurysm.
In a trial randomizing patients to acetylsalicylic acid or coumadin,
the individual presence of a PFO or an atrial septal aneurysm had
no influence on the incidence of recurrent stroke.20 These
differing results on the question of whether a PFO with or without
hypermobility of the septum leads to recurrent stroke are presumably
related to factors (besides patient selection) such as diagnostic
accuracy of the tests and definitions used to identify both the
PFO and the atrial septal aneurysm.
Homma et al25 identified other risk factors: (1) the presence of a Eustachian valve directed toward the PFO (Figure 3); (2) the gaping diameter of the PFO; and (3) the number of micro-bubbles present in the left atrium during the first seconds after release of a Valsalva maneuver during a bubble test.
Catheter-Based PFO Closures
Initial techniques of percutaneous atrial septal defect closure were
documented by King et al in the 1970s,26 Rashkind
in the 1980s,27 and
Sideris et al in the 1990s.28 Bridges
et al29 first proposed
that PFO closure would reduce the incidence of recurrent strokes
and demonstrated a statistically significant effect of PFO closure
on a small group of high-risk patients. Since then, numerous studies
have shown that transcatheter PFO closure with current techniques
is safe and seems to protect against recurrent strokes in this patient
population.30,31 A
randomized trial on the protective effect of transcatheter PFO closure in
recurrent stroke patients has yet to be accomplished.
In 2000, the CardioSEAL device (NMT) and in 2002, the Amplatzer PFO Occluder (AGA) became available for PFO closure in high-risk patients in the United States. At least 5 additional devices have been used clinically abroad.32
The implantation can be performed with a single femoral venous puncture under fluoroscopy without echocardiographic guidance. The PFO can be passed by sliding along the septum primum, coming from the inferior vena cava with a wire or a curved catheter. A transvenous sheath (diameter 3 to 5 mm according to the device selected) is placed in the left atrium. The left-sided disk is unfolded and pulled back against the septum, thereby pulling the septum primum against the septum secundum and closing the slit valve. The right-sided disk is then deployed and the device released. The perfect seat can be assessed before release by echocardiography or by hand-injected dye into the right atrium through the introducer (Figure 6). Follow-up treatment includes acetylsalicylic acid (80 to 300 mg) for a few months, with the addition of clopidogrel (75 mg) or warfarin (International Normalized Ratio 2.5 to 3.5) at some centers. Antibiotics during the interventions are commonplace, and prevention against endocarditis is recommended for a few months until the device is completely covered by tissue.
|
A follow-up TEE after a few months with a tight PFO and no evidence of thrombi on the device signals the cessation of all treatment and controls.
Results With Transcatheter PFO Closure
Technical failures have become extremely rare (for example, inability
to cannulate the PFO is less than 1%). Complications may include
cardiac tamponade, symptomatic air embolism, loss of device, or
puncture site problems; however, none of these have occurred in
the last 400 implantations done by the authors. Complete closure
at follow-up can be expected in 90% to 95% cases with the 2 devices
currently in use. Some trivial residual shunt may be acceptable,
albeit undesirable, as the device will act as a filter for particulate
matter.
Events have recurred in cases where the PFO was not responsible for the index event, in cases where small emboli formed on the left side of the device, or in cases where closure is incomplete.31 In our experience, recurrent events may come close to the natural course for the first year (about 3%), after which they are extremely rare. In contrast, the natural course under platelet inhibitors or warfarin tends to have a steady or even increasing rate of events over the years.21 Hence, the follow-up curves do seem to diverge in favor of device closure in nonrandomized comparisons.
Conclusions and Outlook
Recurrent paradoxical embolism in the presence of a PFO associated with
an atrial septal aneurysm is currently the only unequivocal indication
for PFO closure. A percutaneous attempt should always precede surgical
closure; the latter is unlikely to be rendered more difficult in
case of a failed percutaneous attempt. None of the patients of the
authors in the past 5 years required a surgical intervention. Hence,
surgical PFO closure seems completely supplanted by the percutaneous
approach. This is supported by the fact that recurrence rates for
cerebrovascular accidents or transient ischemic attacks after surgical
closure have been reported as 4%32 to
20%33 per
year.
Because percutaneous closure may take less than 30 minutes under local anesthesia and can be performed as an outpatient procedure with very small risk and inconvenience for the patient, indications are bound to widen, especially if controlled trials and large series confirm that PFO closure reduces the life-long risk of recurrent stroke and perhaps other ailments.
References
- Cohnheim J. Thrombose und Embolie: Vorlesung όber allgemeine Pathologie.
Berlin, Germany: Hirschwald; 1877: 134.
- Windecker S, Meier B. Patent foramen ovale and atrial septal aneurysm:
when and how should they be treated. ACC Curr J Rev. 2002; 11: 97101.
- Steiner MM, Di Tullio MR, Rundek T, et al. Patent foramen ovale
size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;
29: 944948.
[Abstract/Free Full Text]
- Lethen H, Flachskampf FA, Schneider R, et al. Frequency of deep
vein thrombosis in patients with patent foramen ovale and ischemic stroke
or transient ischemic attack. Am J Cardiol. 1997; 80: 10661069.
[CrossRef][Medline]
- Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern
Kentucky Stroke Study: preliminary first-ever and total incidence rates of
stroke among blacks. Stroke. 1998; 29: 415421.
[Abstract/Free Full Text]
- Reul J, Weis J, Jung A, et al. Central nervous system lesions and
cervical disc herniations in amateur divers. Lancet. 1995; 345: 14031405.
[Medline]
- Knauth M, Ries S, Pohimann S, et al. Cohort study of multiple brain
lesions in sport divers: role of a patent foramen ovale. BMJ. 1997;
314: 701705.
[Abstract/Free Full Text]
- Schwerzmann M, Seiler C, Lipp E, et al. Relation between directly
detected patent foramen ovale and ischemic brain lesions in sport divers. Ann
Intern Med. 2001; 134: 2124.
[Medline]
- Wilmshurst PT, Nightingale S, Walsh KP, et al. Effect on migraine
of closure of cardiac right-to-left shunts to prevent recurrence of decompression
illness or stroke or for haemodynamic reasons. Lancet. 2000; 356:
16481651.
[CrossRef][Medline]
- Milhaud D, Bogousslavsky J, van Melle G, et al. Ischemic stroke
and active migraine. Neurology. 2001; 57: 18051811.
[Abstract/Free Full Text]
- Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings
in cryptogenic stroke patients with and without patent foramen ovale: the
PFO-ASA Study. Atrial Septal Aneurysm. Stroke. 2002; 33: 706711.
[Abstract/Free Full Text]
- Sukernik MR, Mets B, Bennett-Guerrero E. Patent foramen ovale
and its significance in the perioperative period. Anesth Analg. 2001;
93: 11371146.
[Free Full Text]
- Konstantinides S, Geibel A, Kasper W, et al. Patent foramen ovale
is an important predictor of adverse outcome in patients with major pulmonary
embolism. Circulation. 1998; 97: 19461951.
[Abstract/Free Full Text]
- Burchell HB, Helmholz HF, Wood EH. Reflex orthostatic dyspnea
associated with pulmonary hypertension. Am J Physiol. 1949; 159: 563564.
- Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events
associated with patent foramen ovale, atrial septal aneurysm, or both. N
Engl J Med. 2001; 345: 17401746.
[Abstract/Free Full Text]
- Overell JR, Bone I, Lees KR. Interatrial septal abnormalities
and stroke: a meta-analysis of case-control studies. Neurology. 2000;
55: 11721179.
[Abstract/Free Full Text]
- Di Tullio M, Sacco RL, Venketasubramanian N, et al. Comparison
of diagnostic techniques for the detection of a patent foramen ovale in stroke
patients. Stroke. 1993; 24: 10201024.
[Abstract]
- Karttunen V, Ventila M, Ikaheimo M, et al. Ear oximetry: a noninvasive
method for detection of patent foramen ovale: a study comparing dye dilution
method and oximetry with contrast transesophageal echocardiography. Stroke. 2001;
32: 448453.
[Abstract/Free Full Text]
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined
cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989; 25: 382390.
[Medline]
- Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment
in stroke patients with patent foramen ovale: Patent Foramen Ovale in Cryptogenic
Stroke study. Circulation. 2002; 105: 26252631.
[Abstract/Free Full Text]
- De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional
characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;
31: 24072413.
[Abstract/Free Full Text]
- Mohr JP, Thompson JLP, Lazar RM, et al. A comparison of warfarin
and aspirin for the prevention of recurrent ischemic stroke. N Engl J
Med. 2001; 345: 14441451.
[Abstract/Free Full Text]
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients
with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic
stroke or transient ischemic attack. French Study Group on Patent Foramen
Ovale and Atrial Septal Aneurysm. Am Heart J. 1995; 130: 10831088.
[Medline]
- Comess KA, De Rook FA, Beach KW, et al. Transesophageal echocardiography
and carotid ultrasound in patients with cerebral ischemia: prevalence of
findings and recurrent stroke risk. J Am Coll Cardiol. 1994; 23: 15981603.
[Medline]
- Homma S, Di Tullio MR, Sacco RL, et al. Characteristics of patent
foramen ovale associated with cryptogenic stroke: a biplane transesophageal
echocardiographic study. Stroke. 1994; 25: 582586.
[Abstract]
- King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal
defect: nonoperative closure during cardiac catheterization. JAMA. 1976;
235: 25062509.
[Abstract]
- Rashkind WJ. Transcatheter treatment of congenital heart disease. Circulation. 1983;
67: 711716.
[Medline]
- Sideris EB, Sideris SE, Fowlkes JP, et al. Transvenous atrial
septal defect occlusion in piglets with a "buttoned" double-disk device. Circulation. 1990;
81: 312318.
[Abstract]
- Bridges ND, Hellenbrand W, Latson L, et al. Transcatheter closure
of patent foramen ovale after presumed paradoxical embolism. Circulation. 1992;
86: 19021908.
[Abstract]
- Windecker S, Wahl A, Chatterjee T, et al. Percutaneous closure
of patent foramen ovale in patients with paradoxical embolism: long-term
risk of recurrent thromboembolic events. Circulation. 2000; 101: 893898.
[Abstract/Free Full Text]
- Wahl A, Meier B, Haxel B, et al. Prognosis after percutaneous
closure of patent foramen ovale for paradoxical embolism. Neurology. 2001;
57: 13301332.
[Abstract/Free Full Text]
- Dearani JA, Ugurlu BS, Danielson GK, et al. Surgical patent foramen
ovale closure for prevention of paradoxical embolism-related cerebrovascular
ischemic events. Circulation. 1999; 100 (suppl II): II171II175.
[Medline]
- Homma S, Di Tullio MR, Sacco RL, et al. Surgical closure of patent
foramen ovale in cryptogenic stroke patients. Stroke. 1997; 28: 23762381.
[Abstract/Free Full Text]